Phone:0591-22985816\22980755
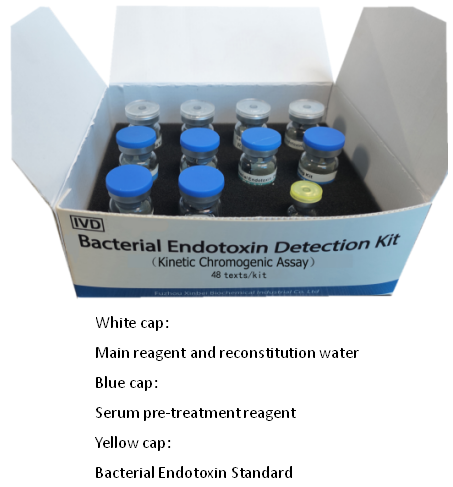
Bacterial Endotoxin Detection Kit (Kinetic Chromogenic Assay)
Certification
CE Registration number: DE/CA20/01-IVD-Luxuslebenswelt-148/21
Intended Use
1. Various systemic infectious diseases;
2. Kidney dialysis patients;
3. Blood station and blood bank;
4. Glam-negative bacterial infection;
5. Fever of undetermined origin;
6. Water and food quality monitoring;
7. Bacterial endotoxin level monitoring in different stage of drug production.
Product features
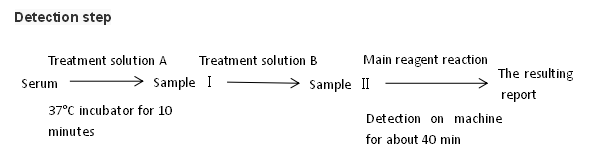
1. It israpid and simple, and the results can be obtained in 1 hour
2. This detection system is only sensitive to endotoxin, the (1-3)-β-D-glucan can not generate false-positive
3. The detection limit of the method is 0.015 Eu/ mL and the linear range is 0.015-0.5 Eu/ mL.
Packaging
48 tests/kit
Previous:
End